APAC is the fast growing region globally for cell & gene therapy trials representing more than a third of all cell & gene studies globally, with China leading in the region.
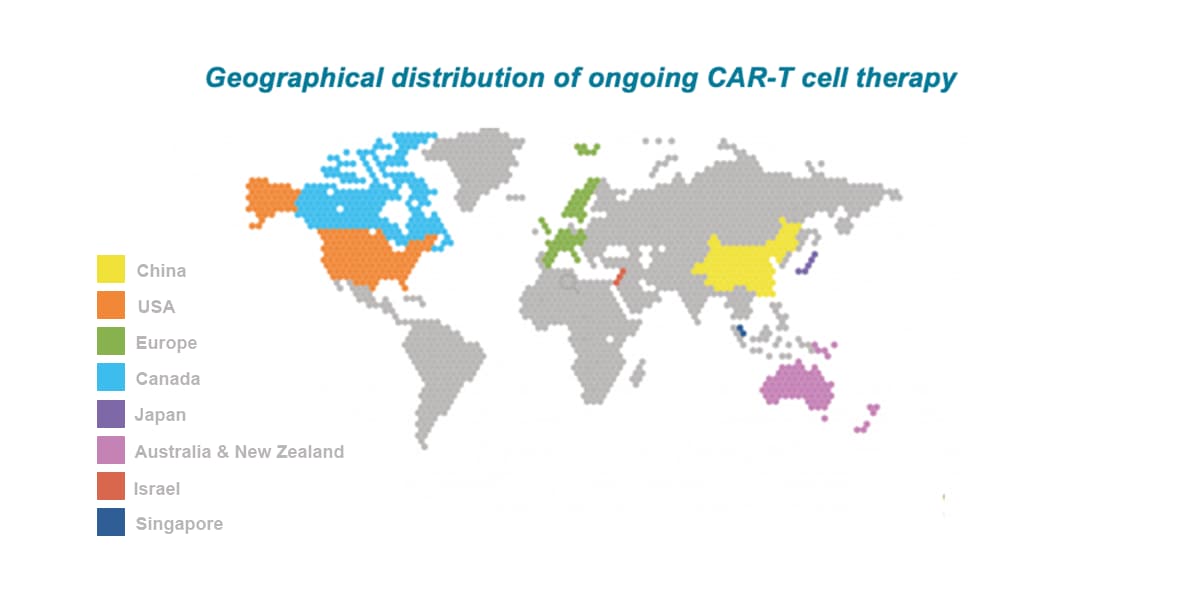
APAC is the leading location globally for CAR-T trials with China attracting ~60% of all CAR-T trials globally between 2015-2022. The number of CAR-T trials initiated by Western companies has rapidly increased in recent years (current CAGR of about 60%), with multiple targets being explored including CD19, CD20, CD22, BCMA, CD30, CD123, CD33, CD38, and CD138.
Novotech, the leading Asia Pacific biotech specialist clinical research organization with operations in APAC and the US, has extensive experience in cell & gene studies.
China has a supportive policy environment promoting a favorable cell and gene therapy ecosystem that includes:
- World-class scientific excellence
- FDA-accepted clinical trial data
- Massive patient population – which facilitates recruitment
- Government support for cell therapy trials with expedited approval processes – shortened the approval timeline to 60 working days
- Development of regional infrastructures such as cell storage and local manufacturing facilities
- Investigator-initiated cell therapy trials, which are widely conducted in China, accumulate valuable data across the different indications
- Top oncology disease treatment facilities in China deliver a practical experience in cell and gene therapy research
- While CAR-T research can be expensive, APAC costs are only about 40-50% of Western countries
- China has the world’s second-largest pharmaceutical market
- Adoption of international regulatory guidelines (ICH)
Patient Recruitment Drivers
The APAC region and specifically China show strong median patient recruitment rates and shorter enrolment periods compared to the US and Europe.
The compelling data around recruitment include:
- China shows enrollment periods 25% shorter than the US and 20% shorter than Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
- China and APAC show 4 times faster median patient recruitment rates than the US and Europe.
- The APAC region shows enrollment periods almost 40% shorter than the US and 35% shorter than Europe
Oncology Cases
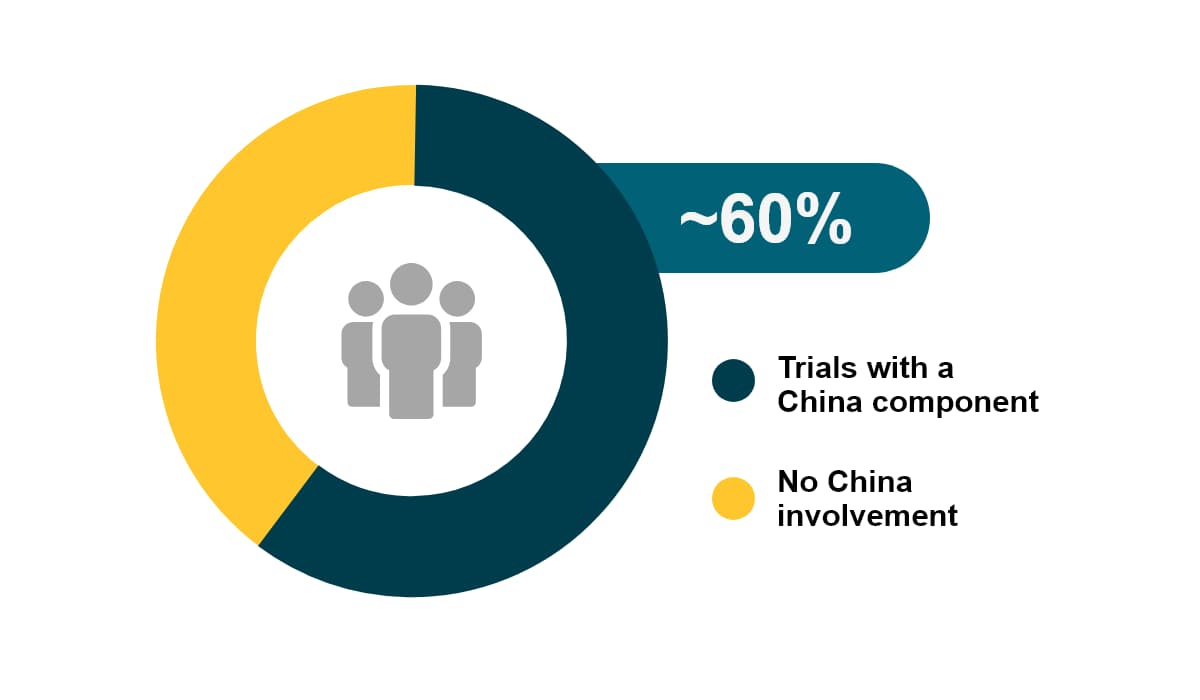
China accounts for 24% of the newly diagnosed cases globally. In 2020 China had about 4.5 million new cancer cases, as well as 30% of cancer-related deaths worldwide. Among all global CAR-T studies, about 60% have China involvement. Over the past 5 years, the growth of oncology trials (CAGR of 25%) in China outpaced all other countries.
“The number of clinical trials in China increased by 20% in 2021 and oncology represented around 40% of all clinical trials, with the majority of these being Phase 1 studies.”
Cell & Gene Therapy Landscape in APAC
Oncology trials occupy the majority of cell & gene therapy trials followed by infectious diseases, CNS, and cardiovascular diseases. Blood cancers, viral infections, and solid tumors are the major oncology indication types in cell and gene therapy trials in the Asia Pacific.
APAC shows nearly 50% faster growth rate in cell & gene therapy trials than the ROW between 2016 and 2021. China shows a 15% faster growth rate than the ROW.
Regionally, China, Australia, Japan, South Korea, and Chinese Taiwan are the leading locations participating in cell & gene therapy trials in APAC.
China Regulatory and IP Spotlight
China continues to align with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), with more than 70% of ICH guidelines now implemented. Importantly also the National Medical Products Administration (NMPA) was re-elected as a member of the ICH Management Committee in June 2021.
In addition, The Center of Drug Evaluation (CDE) guidelines on the clinical development of oncology and rare disease drugs re-emphasize patient-centric, and clinical-value-oriented development on par with ICH.
China also harmonized IP protection with global, with the new patent law effective from June 2021 research organizations.
How to include China in your clinical development strategy

Novotech has just received the AsiaPacific Cell & Gene Excellence Award 2022: Clinical Trials

媒體聯絡人
David James
關於 Novotech Novotech-CRO.com
Novotech成立於 1997 年,立足亞太,面向全球,是專業的生物技術合同研究組織(CRO)。
Novotech因其在行業內的突出貢獻而備受讚譽,曾榮獲多項殊榮,其中包括 2023 年 CRO 領導力獎 (CRO Leadership Award 2023) 、2023 年亞太地區細胞與基因治療臨床試驗卓越獎 (Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023)和自 2006 年以來蟬聯亞太地區合同研究組織年度公司獎 (Asia-Pacific Contract Research Organization Company of the Year Award)。
Novotech是一家包含實驗室、Ⅰ期臨床中心、藥物開發諮詢服務和專業FDA法規服務的臨床CRO,擁有超5,000 項臨床專案經驗,包括Ⅰ期至Ⅳ期臨床試驗。Novotech專注於服務在亞太、美國與歐洲等地進行臨床試驗的生物技術客戶。Novotech目前在全球34個辦公地點共擁有3000多名員工。
如欲了解更多資訊或與專家團隊成員交談,請造訪 www.Novotech-CRO.com







